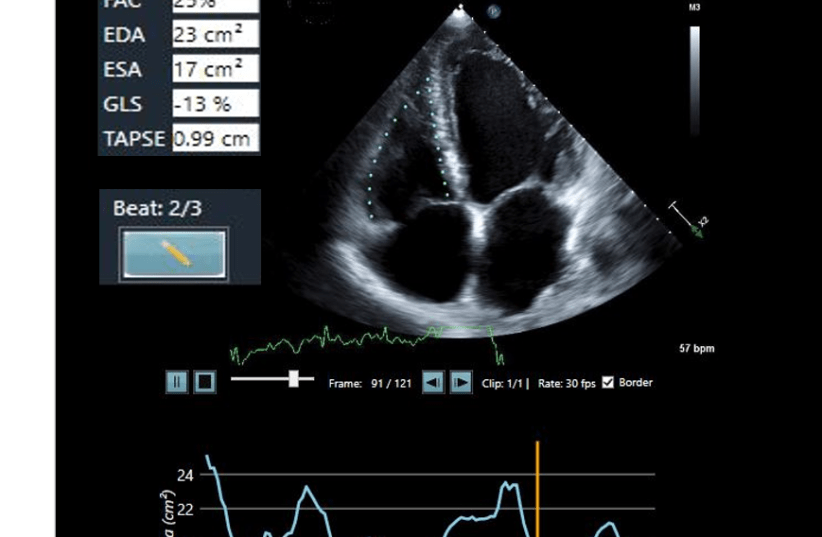
Israeli AI leader DiA receives FDA clearance for ultrasound solutions
The addition of two new FDA clearances to DiA's LVivo toolbox reflects changes in the MedTech industry, with ultrasounds and AI-based analysis solutions becoming more valued by healthcare workers.